A carbocation is really a positively charged carbon ion which includes 6 electrons in its valence shell instead of the usual 8...
A further method for finding the end issue is always to plot the very first spinoff with the titration curve, which supplies its slope at Every single stage together the x
At the end of the demonstration, the teacher will also clarify the way to work out the molarity from the unknown material. Grade Stage
The indicator used for this intent can be phenolphthalein which kinds pink colour in primary Resolution which is colourless in acid and neutral Option.
Early samples of acid–base titrimetry contain deciding the acidity or alkalinity of answers, and identifying the purity of carbonates and alkaline earth oxides.
Taking the unfavorable log of both sides of Equation ref 9.5 , and rearranging to unravel for pH leaves us using a equation that relates the answer’s pH for the relative concentrations of HIn As well as in–.
Titrations can even be accustomed to furnish the purity of samples, calculation with regards to PH, etcetera. Any calculations in the Examination can be done in two strategies.
Sooner or later, all of the acetic acid is consumed. Addition of even a portion of a fall of titrant produces a long-lasting pink colour resulting from unreacted NaOH within the flask. The color modify that happens within the endpoint from the indicator indicators that every one the acetic acid is consumed, so Now we have arrived at the equivalence point in the titration.
The titration curve in an acid-base titration represents the strength of your matching acid and base. The curve will be to some degree easy and rather steep close to the equivalence place for a powerful acid and a robust base.
Any of the three indicators will show a reasonably sharp coloration alter for the equivalence position with the potent acid titration, but only phenolphthalein is suitable for use during the weak acid titration.
a with the fifty percent-equivalence issue more info system overestimates its value Should the acid is just too powerful and underestimates its benefit If your acid is too weak.
, by way of example, reveals a series of titration curves to the titration of several concentrations of HCl with equimolar answers NaOH. For titrand and titrant concentrations lesser than ten–three M, the transform in pH at the tip point is simply too small to provide an precise and a here exact final result.
Bases are of two types: solid and weak. The identical course of action is finished in the situation of acid titration apart from which the unknown Alternative (titrate) is the base and titrant is a strong acid.
As observed while in the chapter to the stoichiometry of chemical reactions, titrations may be used to quantitatively assess remedies for his or her acid or base concentrations. During this portion, We're going to examine the fundamental chemical equilibria that make acid-base titrimetry a helpful analytical method.
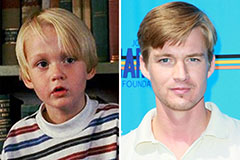 Mason Gamble Then & Now!
Mason Gamble Then & Now! Shannon Elizabeth Then & Now!
Shannon Elizabeth Then & Now! Batista Then & Now!
Batista Then & Now! Dawn Wells Then & Now!
Dawn Wells Then & Now! Megyn Kelly Then & Now!
Megyn Kelly Then & Now!